Biogeochemical cycles / Nutrient
cycles
The cycling of chemicals between the biological and the geological world is
called Biogeochemical cycle.
The Biotic and Abiotic components of the biosphere constantly interact through
biogeochemical cycles. During these interactions, there is a transfer of
nutrients between living organisms and the non-living environment.
The important biogeochemical cycles are water cycle, Nitrogen cycle, Carbon cycle
and Oxygen cycle, Phosphorus cycle and Sulphur cycle.
Biogeochemical Cycles are classified into :
1. Atmospheric cycles - Ex: Carbon, Oxygen and Nitrogen cycles
2. Hydrological cycle - Ex: Water cycle
3. Sedimentary cycle - Ex: Phosphorus and Sulphur cycles
Biogeochemical Cycles are classified into :
1. Atmospheric cycles - Ex: Carbon, Oxygen and Nitrogen cycles
2. Hydrological cycle - Ex: Water cycle
3. Sedimentary cycle - Ex: Phosphorus and Sulphur cycles
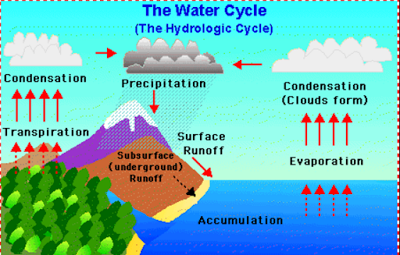
The water cycle involves various steps like evaporation, transpiration,
condensation and precipitation.
• When the water bodies are heated during the day,
water enters the atmosphere as water vapour by the process of evaporation.
• There is another way in which water evaporates
into the atmosphere. This happens through transpiration.
• The water vapours in the atmosphere changes to
water droplets and collects to form clouds. This process is called condensation.
• Air currents move the clouds formed by
condensation and carry them over the land, where they break into rain, snow or
fog. This is called precipitation.
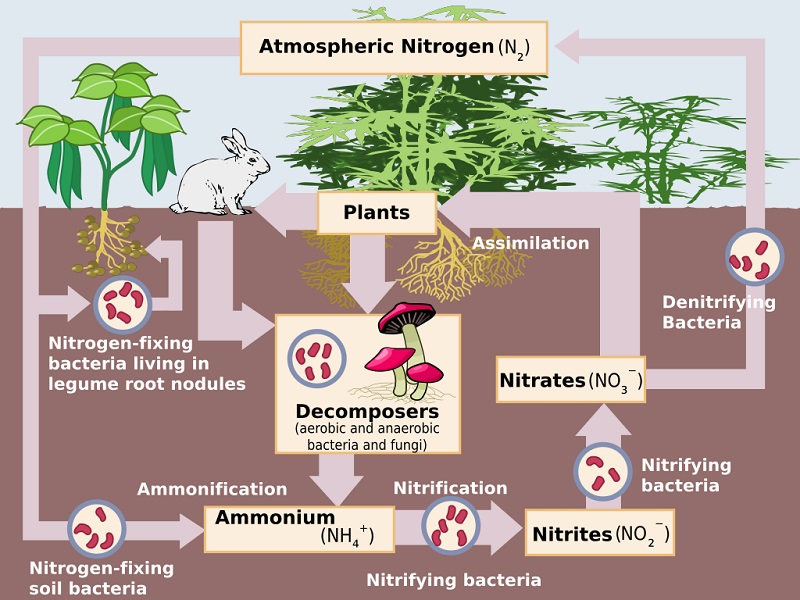
The sequence in which nitrogen passes from the atmosphere to the soil and
organisms, and then is eventually released back into the atmosphere, is called
the Nitrogen cycle.
• Nitrogen makes up 78 percent of the earth’s
atmosphere. The percentage of nitrogen in the atmosphere is maintained by
nitrogen cycle.
• Nitrogen is an essential constituent of proteins,
nucleic acids like DNA and RNA, vitamins, and chlorophyll.
• Plants and animals cannot utilise atmospheric
nitrogen readily. It has to be fixed by some organisms called as nitrogen
fixers.
• Nitrogen-fixing bacteria live in the root nodules of
certain leguminous plants.. These bacteria convert atmospheric nitrogen into
ammonia, which is utilised readily by plant called Nitrogen fixation.
• Nitrogen-fixing bacteria along with free living
bacteria in the soil achieve 90 percent of nitrogen fixation.
• Lightning plays an important role in nitrogen
fixation. When lightning occurs, the high temperature and pressure convert
nitrogen and water into nitrates and nitrites.Nitrates and nitrites dissolve in water and are
readily used by aquatic plants and animals.
• Ammonification is the process by which soil bacteria decompose dead organic
matter and release ammonia into the soil.
• Nitrification
is the process by which ammonia is converted into nitrites and nitrates.
• Denitrification
is the process by which nitrates are converted into atmospheric nitrogen.
Carbon
cycle
Carbon is cycled repeatedly through different forms by the various physical and
biological activities constituting the carbon cycle.
Carbon cycle maintains the balance of the element carbon in the atmosphere.
Carbon is found in various forms on the Earth.
*Diamond and graphite found in the soil are made up of an element called
carbon.
*Carbon is present in the atmosphere as carbon dioxide.
*Carbon can also occur as carbonates and bicarbonate salts in minerals. The
endoskeletons and exoskeletons of various aquatic animals are also formed from
carbonate salts.
*Carbon is an essential part of nutrients like carbohydrates, fats, proteins,
nucleic acids and vitamins.
Carbon cycle maintains the amount of carbon in the atmosphere. The carbon cycle
starts in plants.
Step - 1 : Plants, use carbon dioxide in the atmosphere, convert it into glucose in
the presence of sunlight by the process of photosynthesis. Plants and animals
break these carbohydrates for energy and release carbon dioxide through
respiration.Step - 2 : When the plants and animals die, fungi and bacteria decompose the dead remains. This releases the carbon in the remains as carbon dioxide.
Step - 3 : Some plants and animals which get burried in the soil under certain temperature and pressure over millions of years get transformed into fossil fuels. Coal and petroleum are some of the fossil fuels. On burning these fuels, carbon dioxide is released into the atmosphere.

Oxygen cycle
The sequence in which oxygen from the atmosphere is used by organisms and
eventually released back into the atmosphere through photosynthesis is called
as oxygen cycle.
• Oxygen makes up 21 percent of the air. It is an
essential constituent of carbohydrates, proteins, fats and nucleic acids.
• Oxygen is found in air, in combined form as carbon
dioxide, and in the earth’s crust as carbonates, sulphates and nitrates.
• Plants and animals use atmospheric oxygen during
respiration and release the same during photosynthesis.
• Fossil fuels require oxygen for combustion.
• The ozone layer is present in stratosphere, one of
the layers of the atmosphere. Each molecule of ozone is made up of three oxygen
atoms. The ozone layer prevents harmful radiations from reaching the earth’s
surface, where they might damage life forms.

Phosphorus cycle
Phosphorous is an essential nutrient found in the macromolecules
of humans and other organisms, including DNA
· The phosphorous cycle is slow. Most phosphorous in nature exists
in the form of phosphate ion. Phosphorus is often the limiting nutrient, or
nutrient that is most scarce and thus limits growth, in aquatic ecosystems.
· When nitrogen and phosphorous from fertilizer are carried in
runoff to lakes and oceans, they can cause eutrophication, the
overgrowth of algae. The algae may deplete oxygen from the water and create
a dead zone.
In nature, phosphorous
is found mostly in the form of phosphate ions. Phosphate compounds are found in
sedimentary rocks, and as the rocks weather wear down over long time periods the
phosphorous they contain slowly leaches into surface water and soils. Volcanic
ash, aerosols, and mineral dust can also be significant phosphate sources.
Phosphate compounds in
the soil can be taken up by plants and, from there, transferred to animals that
eat the plants. When plants and animals excrete wastes or die, phosphates are
returned to the soil. Phosphorous-containing compounds may also be carried in
surface runoff to rivers, lakes, and oceans, where they are taken up by aquatic
organisms.
When
phosphorous-containing compounds from the bodies or wastes of marine organisms
sink to the floor of the ocean, they form new sedimentary layers. Over long
periods of time, phosphorous-containing sedimentary rock may be moved from the
ocean to the land by a geological process called uplift. However, this process
is very slow, and the average phosphate ion has an oceanic residence time in
the ocean of 20,000 to 100,000 years.

Sulphur Cycle:
Sulphur is one of the
components that make up proteins and vitamins. Proteins consist of amino acids
that contain sulphur atoms. Sulphur is important for the functioning of
proteins and enzymes in plants, and in animals that depend upon plants for
sulphur.
It enters the atmosphere through both natural and human sources.
Natural recourses can be for instance volcanic eruptions, bacterial processes,
evaporation from water, or decaying organisms. When sulphur enters the
atmosphere through human activity, this is mainly a consequence of industrial
processes where sulphur dioxide (SO2) and hydrogen
sulphide (H2S) gases are emitted on a wide scale.
When sulphur dioxide enters the atmosphere it will react with
oxygen to produce sulphur trioxide gas
(SO3), or with other chemicals in the atmosphere, to
produce sulphur salts. Sulphur dioxide may also react with water to produce sulphuric acid (H2SO4). Sulphuric
acid may also be produced from demethyl-sulphide,
which is emitted to the atmosphere by plankton species.
All these particles
will settle back onto earth, or react with rain and fall back onto earth as
acid deposition. The particles will then be absorbed by plants again and are
released back into the atmosphere, so that the sulphur cycle will start over
again.










0 comments: